A battery is an electrochemical device that stores and releases electrical energy using chemical reactions. A battery is made up of one or more electrochemical cells. An electrochemical cell or battery cell consists of a positive electrode (cathode) which accepts electrons, negative electrode (anode) which generates electrons, and an electrolyte which allows ions, but not electrons, to travel between the electrodes. During the discharge of the electrochemical system, the anode undergoes an oxidation reaction which releases electrons. These electrons travel to the cathode which undergoes a reduction reaction (gaining of electrons) and flow out of the battery to provide electric current through an external circuit.
The chemical reaction that produces electric current is either one-shot or reversible. When the anode has been fully oxidized, the battery runs out. Batteries that have a chemical reaction proceeding in one direction and are used only once are called primary batteries. Batteries that utilize reversible chemical reactions to store and discharge energy for multiple cycles are referred to as rechargeable batteries. In a rechargeable battery, the electrochemical process that occurs during discharge can be reversed by passing an electric current through the cell in the opposite direction. Rechargeable batteries are commonly classified by their chemistries. Some of the chemistries commonly used in rechargeable batteries are lithium-ion (Li-ion), and nickel metal hydride (NiMH).
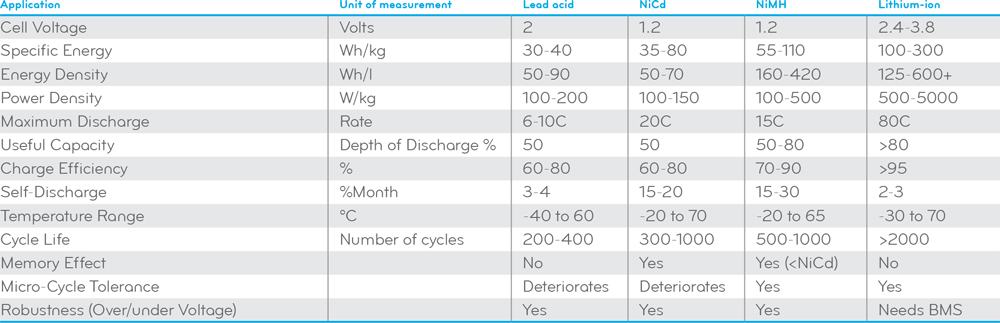
[H2]Lead-acid Battery[/H2]Lead-acid batteries use a lead dioxide (PbO2) as the cathode and lead (Pb) as the anode. In the discharge state these active materials react with the sulphuric acid electrolyte (H2SO4 and H2O) to produce lead sulfate (PbSO4) and electrons according to this chemical reaction: PbO2 + Pb + 2H2SO4 = 2PbSO4 + 2H2O. In the charge state, the cycle is reversed. The lead sulfate gets dissociated and re-deposited on the electrodes. The material on the positive electrode is converted to lead dioxide, the material on the negative electrode is converted to lead. Sulfuric acid is produced at both electrodes and water is consumed at the positive electrode.
There are two main families of lead-acid batteries divided by construction type. Flooded lead acid batteries, sometimes referred to as vented lead-acid batteries, have their plates submerged in the liquid sulphuric acid electrolyte and requires regular topping up with distilled water. Sealed lead-acid batteries have essentially the same chemistry as flooded lead-acid batteries but use a sealed design and an immobilized electrolyte. This construction allows sealed lead-acid batteries to retain their electrolyte internally regardless of orientation. The need for regular battery maintenance ((addition of water and electrolyte stratification) is also reduced. The electrolyte of sealed lead-acid batteries is immobilized by either soaking it in an absorptive separator consisting of matted glass fibers, as in absorbed glass mat (AGM) lead-acid batteries, or by forming a gel through the addition of silica, as in gel lead-acid batteries. Both types are valve regulated lead-acid (VRLA) batteries because they use a resealable low pressure safety valve to eliminate excess pressure from the chemical reactions when gases are generated during charging or overcharging.
Advantages
Lead-acid batteries are the cheapest energy storage solution of the all the technologies currently available on the market. Lead-acid batteries can be designed to deliver a short burst of high power for use in automotive SLI (Starting, Lighting and Ignition) applications. Lead-acid batteries can also be designed as deep-cycle batteries to deliver a lower, steady level of power for a much longer time, making it a reliable, cost-effective option for providing emergency backup power for telecommunications and computer centers; for use in minor micro-grids or the grid-independent electrical power systems, uninterrupted power supply and spinning reserve applications; and for driving wheelchairs, forklifts, golf carts, and electric scooters and bicycles.
Disadvantages
Lead-acid batteries have a low specific energy (30-40 Wh/kg) and energy density (50-90 Wh/l). Their short cycle life (200-400 cycles) means frequent replacement and high maintenance costs. Not only lead-acid batteries have a low charge efficiency (60-80%), their long charging time (10-24 hours) significantly compromises the flexibility of use. Thermal runaway may occur if the battery is incorrectly charged or not properly thermally managed. Lead-acid batteries must be recycled since it contains harmful substances such as lead, antimony, arsenic and sulphuric acid. Storage in a discharged state for long term irreversibly damages the battery’s electrodes.
[H2]Lithium-ion Battery[/H2]A lithium-ion (Li-ion) battery utilizes an intercalated lithium compound as electrode material. Lithium ions that are incorporated into the structure of the electrode materials move from the cathode to the anode electrode during charging and from the anode to the cathode as the battery is discharged. Lithium ions move through a separator which is typically made of a polymeric membrane saturated with a liquid electrolyte. The electrolyte system facilitates lithium ion transport but prevents direct contact between the electrodes, and prevents growth of dendrites (metal slivers) between the electrodes.
Lithium-ion batteries are often classified by their cathode chemistry. Five major types of cathode materials commercially available on the market are LCO (lithium cobalt oxide), LFP (lithium-iron phosphate), NCA (lithium nickel cobalt aluminum oxide), NCM (lithium nickel cobalt manganese oxide), and LMO (lithium manganese oxide). The anode in today's lithium-ion batteries is commonly made of a carbon compound (i.e. graphite) which has a high capacity and a very light weight. Another one which is of interest is the LTO (lithium titanate oxide) which can enable extremely fast charging. On the downside, this material is expensive and has low energy capacity and high voltage versus lithium ions.
Advantages
Lithium-ion batteries offer the highest energy densities (125-600+ Wh/l), specific energy (100-300 Wh/kg), charge efficiency (>95%) as well as the lowest weight of all battery chemistries. Lithium-ion has a long cycle life of up to 3000 cycles at 80% depth-of-discharge (DoD). Its cost effectiveness in hot climates is comparable to lead-acid batteries because of their high temperature stability. Lithium-ion batteries will remain stable until temperatures routinely exceed 49°C whereas high ambient temperature can significantly affect the cycle life of lead acid (flooded and VRLA) batteries which drops to 50% of its moderate climate rating. Lithium-ion batteries do not suffer from the high self-discharge rate and memory effect of NiCd and NiMH batteries. All these advantages make lithium-ion batteries the battery of choice for smartphones, digital cameras, tablet computers and off-grid products. Li-ion technology exhibits many qualities for off-grid applications and has the lion’s share in grid storage. These qualities also keep Li-ion as a dominant battery chemistry in plug-in hybrid-electric vehicles (HEVs) and full electric vehicles (EVs) for years to come.
Disadvantages
Lithium-ion batteries tend to have one of the highest cost-per-watt ratios of all battery technologies. The likelihood and consequences of thermal runaway are higher and more damaging for lithium-ion batteries as these batteries have a higher amount of energy in a smaller volume and use a flammable organic electrolyte. Physical damages, improper operation, electrical abuse such as short circuits and overcharging, and elevated temperature reactions between electrodes and electrolyte can cause a thermal runaway. Li-ion cells should always be monitored and controlled by a battery management system (BMS) so that thermal runaway caused by electrical abuse does not cause the battery to light on fire and/or explode. High temperature charging (>45°C) or low temperature charging (<0°C) should be avoided for lithium-ion batteries.
[H2]Nickel Metal Hydride Battery[/H2]Nickel metal hydride (NiMH) were originally developed as a replacement for nickel cadmium (NiCd) batteries which use the highly toxic substance cadmium as the anode. The NiMH battery uses nickel hydroxide for the cathode. The anode is a hydrogen-storing metal alloy made from a metal hydride, usually alloys of lanthanum and rare earths capable of absorbing hydrogen equivalent to about a thousand times of their own volume. The electrolyte is alkaline, usually potassium hydroxide. Under charging nickel hydroxide is converted to nickel oxyhydroxide while the hydrogen-storing alloy absorbs hydrogen from the electrolyte to form a metal hydroxide. During the discharge of the electrochemical system, nickel oxyhydroxide reacts with water to produce the nickel hydroxide and hydroxide ion at the anode.
Advantages
NiMH batteries are capable of storing twice or three times the energy as lead-acid batteries for the same weight. NiMH batteries have a cycle life (500-1000 cycles) and an energy density (160-420 Wh/l) only second to lithium-ion batteries. The power density of NiMH batteries can be as high as 800+ W/kg, which enables them to be rapidly charged. NiMH batteries are an upgraded option for many applications which traditionally would have used NiCd batteries but suffer less from memory effect and are more cost effective to recycle than NiCd batteries. NiMH was once the technology of choice in the plug-in HEV and full EV market due to its better safety properties over lithium-ion batteries as well as its higher capacity, energy density, charge efficiency, and micro-cycle tolerance over lead-acid batteries.
Disadvantages
NiMH batteries are still significantly more expensive than lead-acid batteries and cannot compete with lithium-ion batteries when it comes to battery capacity, energy density, cycle life, and charging performance. Although the memory effect is less of an issue with NiMH chemistry than in nickel cadmium, it must be used periodically to prevent the memory effect typically associated with shallow discharge/charge modes. NiMH batteries have not been considered for use in SLI applications in vehicles because of their inferior cold-cranking performance. NiMH chemistry generates more heat than nickel cadmium and thus requires controlled charging (including effective charge termination) to prevent the battery from overheating and being damaged.
Source: Johnson Matthey Battery Systems
The chemical reaction that produces electric current is either one-shot or reversible. When the anode has been fully oxidized, the battery runs out. Batteries that have a chemical reaction proceeding in one direction and are used only once are called primary batteries. Batteries that utilize reversible chemical reactions to store and discharge energy for multiple cycles are referred to as rechargeable batteries. In a rechargeable battery, the electrochemical process that occurs during discharge can be reversed by passing an electric current through the cell in the opposite direction. Rechargeable batteries are commonly classified by their chemistries. Some of the chemistries commonly used in rechargeable batteries are lithium-ion (Li-ion), and nickel metal hydride (NiMH).
[H2]Lead-acid Battery[/H2]Lead-acid batteries use a lead dioxide (PbO2) as the cathode and lead (Pb) as the anode. In the discharge state these active materials react with the sulphuric acid electrolyte (H2SO4 and H2O) to produce lead sulfate (PbSO4) and electrons according to this chemical reaction: PbO2 + Pb + 2H2SO4 = 2PbSO4 + 2H2O. In the charge state, the cycle is reversed. The lead sulfate gets dissociated and re-deposited on the electrodes. The material on the positive electrode is converted to lead dioxide, the material on the negative electrode is converted to lead. Sulfuric acid is produced at both electrodes and water is consumed at the positive electrode.
There are two main families of lead-acid batteries divided by construction type. Flooded lead acid batteries, sometimes referred to as vented lead-acid batteries, have their plates submerged in the liquid sulphuric acid electrolyte and requires regular topping up with distilled water. Sealed lead-acid batteries have essentially the same chemistry as flooded lead-acid batteries but use a sealed design and an immobilized electrolyte. This construction allows sealed lead-acid batteries to retain their electrolyte internally regardless of orientation. The need for regular battery maintenance ((addition of water and electrolyte stratification) is also reduced. The electrolyte of sealed lead-acid batteries is immobilized by either soaking it in an absorptive separator consisting of matted glass fibers, as in absorbed glass mat (AGM) lead-acid batteries, or by forming a gel through the addition of silica, as in gel lead-acid batteries. Both types are valve regulated lead-acid (VRLA) batteries because they use a resealable low pressure safety valve to eliminate excess pressure from the chemical reactions when gases are generated during charging or overcharging.
Advantages
Lead-acid batteries are the cheapest energy storage solution of the all the technologies currently available on the market. Lead-acid batteries can be designed to deliver a short burst of high power for use in automotive SLI (Starting, Lighting and Ignition) applications. Lead-acid batteries can also be designed as deep-cycle batteries to deliver a lower, steady level of power for a much longer time, making it a reliable, cost-effective option for providing emergency backup power for telecommunications and computer centers; for use in minor micro-grids or the grid-independent electrical power systems, uninterrupted power supply and spinning reserve applications; and for driving wheelchairs, forklifts, golf carts, and electric scooters and bicycles.
Disadvantages
Lead-acid batteries have a low specific energy (30-40 Wh/kg) and energy density (50-90 Wh/l). Their short cycle life (200-400 cycles) means frequent replacement and high maintenance costs. Not only lead-acid batteries have a low charge efficiency (60-80%), their long charging time (10-24 hours) significantly compromises the flexibility of use. Thermal runaway may occur if the battery is incorrectly charged or not properly thermally managed. Lead-acid batteries must be recycled since it contains harmful substances such as lead, antimony, arsenic and sulphuric acid. Storage in a discharged state for long term irreversibly damages the battery’s electrodes.
[H2]Lithium-ion Battery[/H2]A lithium-ion (Li-ion) battery utilizes an intercalated lithium compound as electrode material. Lithium ions that are incorporated into the structure of the electrode materials move from the cathode to the anode electrode during charging and from the anode to the cathode as the battery is discharged. Lithium ions move through a separator which is typically made of a polymeric membrane saturated with a liquid electrolyte. The electrolyte system facilitates lithium ion transport but prevents direct contact between the electrodes, and prevents growth of dendrites (metal slivers) between the electrodes.
Lithium-ion batteries are often classified by their cathode chemistry. Five major types of cathode materials commercially available on the market are LCO (lithium cobalt oxide), LFP (lithium-iron phosphate), NCA (lithium nickel cobalt aluminum oxide), NCM (lithium nickel cobalt manganese oxide), and LMO (lithium manganese oxide). The anode in today's lithium-ion batteries is commonly made of a carbon compound (i.e. graphite) which has a high capacity and a very light weight. Another one which is of interest is the LTO (lithium titanate oxide) which can enable extremely fast charging. On the downside, this material is expensive and has low energy capacity and high voltage versus lithium ions.
Advantages
Lithium-ion batteries offer the highest energy densities (125-600+ Wh/l), specific energy (100-300 Wh/kg), charge efficiency (>95%) as well as the lowest weight of all battery chemistries. Lithium-ion has a long cycle life of up to 3000 cycles at 80% depth-of-discharge (DoD). Its cost effectiveness in hot climates is comparable to lead-acid batteries because of their high temperature stability. Lithium-ion batteries will remain stable until temperatures routinely exceed 49°C whereas high ambient temperature can significantly affect the cycle life of lead acid (flooded and VRLA) batteries which drops to 50% of its moderate climate rating. Lithium-ion batteries do not suffer from the high self-discharge rate and memory effect of NiCd and NiMH batteries. All these advantages make lithium-ion batteries the battery of choice for smartphones, digital cameras, tablet computers and off-grid products. Li-ion technology exhibits many qualities for off-grid applications and has the lion’s share in grid storage. These qualities also keep Li-ion as a dominant battery chemistry in plug-in hybrid-electric vehicles (HEVs) and full electric vehicles (EVs) for years to come.
Disadvantages
Lithium-ion batteries tend to have one of the highest cost-per-watt ratios of all battery technologies. The likelihood and consequences of thermal runaway are higher and more damaging for lithium-ion batteries as these batteries have a higher amount of energy in a smaller volume and use a flammable organic electrolyte. Physical damages, improper operation, electrical abuse such as short circuits and overcharging, and elevated temperature reactions between electrodes and electrolyte can cause a thermal runaway. Li-ion cells should always be monitored and controlled by a battery management system (BMS) so that thermal runaway caused by electrical abuse does not cause the battery to light on fire and/or explode. High temperature charging (>45°C) or low temperature charging (<0°C) should be avoided for lithium-ion batteries.
[H2]Nickel Metal Hydride Battery[/H2]Nickel metal hydride (NiMH) were originally developed as a replacement for nickel cadmium (NiCd) batteries which use the highly toxic substance cadmium as the anode. The NiMH battery uses nickel hydroxide for the cathode. The anode is a hydrogen-storing metal alloy made from a metal hydride, usually alloys of lanthanum and rare earths capable of absorbing hydrogen equivalent to about a thousand times of their own volume. The electrolyte is alkaline, usually potassium hydroxide. Under charging nickel hydroxide is converted to nickel oxyhydroxide while the hydrogen-storing alloy absorbs hydrogen from the electrolyte to form a metal hydroxide. During the discharge of the electrochemical system, nickel oxyhydroxide reacts with water to produce the nickel hydroxide and hydroxide ion at the anode.
Advantages
NiMH batteries are capable of storing twice or three times the energy as lead-acid batteries for the same weight. NiMH batteries have a cycle life (500-1000 cycles) and an energy density (160-420 Wh/l) only second to lithium-ion batteries. The power density of NiMH batteries can be as high as 800+ W/kg, which enables them to be rapidly charged. NiMH batteries are an upgraded option for many applications which traditionally would have used NiCd batteries but suffer less from memory effect and are more cost effective to recycle than NiCd batteries. NiMH was once the technology of choice in the plug-in HEV and full EV market due to its better safety properties over lithium-ion batteries as well as its higher capacity, energy density, charge efficiency, and micro-cycle tolerance over lead-acid batteries.
Disadvantages
NiMH batteries are still significantly more expensive than lead-acid batteries and cannot compete with lithium-ion batteries when it comes to battery capacity, energy density, cycle life, and charging performance. Although the memory effect is less of an issue with NiMH chemistry than in nickel cadmium, it must be used periodically to prevent the memory effect typically associated with shallow discharge/charge modes. NiMH batteries have not been considered for use in SLI applications in vehicles because of their inferior cold-cranking performance. NiMH chemistry generates more heat than nickel cadmium and thus requires controlled charging (including effective charge termination) to prevent the battery from overheating and being damaged.
Source: Johnson Matthey Battery Systems



